
- Dreamstime
- Pfizer logo
“I’m a science guy; I have to research and stuff,” said Tolle, 63. “And I do gardening. I'm outside a lot more since I retired.”
The two-year research project was being conducted on behalf of Pfizer, a $100 billion global pharmaceutical company. Participants were told it involved being injected with several doses of an experimental vaccine against Lyme disease, a tick-borne malady that causes an array of symptoms, some serious.
Related Data Dive: Vermont Has the Nation's Highest Lyme Disease Rate. Where Does Your County Rank?
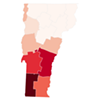
Data Dive: Vermont Has the Nation's Highest Lyme Disease Rate. Where Does Your County Rank?
Off Message
Tolle agreed, and in November, he arrived at an office building near Middlebury for his first appointment. After a few hours in the waiting room with fellow volunteers, he filled out a mountain of paperwork and got his shot.
Tolles’ second visit to the clinic was scheduled for a Sunday in early January. On the Friday before, he said, he got a call confirming the time of his visit, but on Saturday, another call informed him it was off.
“They said, ‘Be sure to see your health provider if you have any concerns about your health, or contact us. But don’t come, we’ll reschedule,'" he said.
The company handling the vaccination clinics, Care Access, never called back to reschedule, and Tolles and others who had signed up were left to wonder. A Nantucket newspaper published a story about the abrupt cancellation of the Lyme study there, and a few people took to social media to express their puzzlement.
Some questions were answered when Pfizer sent out a press release on February 17 explaining that it had decided to drop about half the participants in its huge national study called VALOR, or Vaccine Against Lyme for Outdoor Recreationists, because of problems with the clinical practices of the vendor.
Pfizer declined in an email on Wednesday to disclose how many Vermonters had signed up for the trial or to provide any other details.
The pharmaceutical giant reassured participants in a letter that there were no safety concerns and that the study hadn’t dropped them as a result of volunteers reporting adverse reactions to the vaccine. However, the situation left Tolle uneasy.
Tick-borne Lyme disease, first diagnosed in the region of Lyme, Conn., in 1975, is a big problem in Vermont. The state was No. 2 per capita for incidence of the disease in 2021, according to the journal Clinical Advisor, after New Jersey. Maine, Rhode Island and Connecticut are also in the top five.
Diagnoses have risen by 60 percent in rural areas and 19 percent in urban areas over the past five years, the journal reported in August. Infection can cause fever, headache, fatigue and skin rash. Left untreated, it can affect the joints, the heart and the nervous system, according to the U.S. Centers for Disease Control and Prevention.
Researchers are trying to develop a vaccine that can protect people. Tolle wanted to be a part of that, he said; he'd met a woman through his volunteer work whose son died of complications from Lyme disease.
“That made me really want to support this potential solution to the problem,” he said. He also got a $60 gift card as reimbursement for his first visit.
Pfizer said its clinical trial is continuing at other sites — though with a different contractor — and that it’s still enrolling new participants.
For its part, Care Access, the contractor that set up some of the mobile clinics, said it disagreed with Pfizer’s decision to drop them. In a press release, the company said it is committed to safety and research excellence.
“We are sharing information with the FDA and the independent Institutional Review Board for this study to ensure they have the facts,” the company said. “We are most disappointed for the study participants and are heartbroken about the impact this will have on the underserved communities that we reached and with whom we partnered.”
Related The Bite of a Lone Star Tick Can Cause Alpha-Gal Syndrome, an Allergy to Meat and Dairy

The Bite of a Lone Star Tick Can Cause Alpha-Gal Syndrome, an Allergy to Meat and Dairy
Health + Fitness
“I'm sure I'm not alone in wanting to know more about why the study was canceled suddenly and what the problem was with the way Care Access was conducting their business,” Tolles said.






Comments
Comments are closed.
From 2014-2020, Seven Days allowed readers to comment on all stories posted on our website. While we've appreciated the suggestions and insights, right now Seven Days is prioritizing our core mission — producing high-quality, responsible local journalism — over moderating online debates between readers.
To criticize, correct or praise our reporting, please send us a letter to the editor or send us a tip. We’ll check it out and report the results.
Online comments may return when we have better tech tools for managing them. Thanks for reading.